The Review Choice Demonstration (RCD) for Home Health Services is coming soon to Illinois and will later expand to Ohio, North Carolina, Texas, and Florida.
While the industry awaits a final word from CMS on the effective date for Illinois, we are pleased to announce that the following software updates have been released in preparation for RCD:
Axxess has released software enhancements that allow agencies using pre-claim review (PCR) to upload and store all required documents in one location and assign each claim’s Unique Tracking Number (UTN) to automatically append to all Medicare final claims.
The ability to manage PCR documents is permission-based, so administrators (or other agency leadership) must identify which users should have this permission and assign accordingly in the system.
All agencies in Illinois have automatic access to give users PCR permission but can disable this ability if they choose, by selecting the Admin tab➜ Lists➜ Insurances/Payors and selecting Edit Visit Rate next to Medicare.
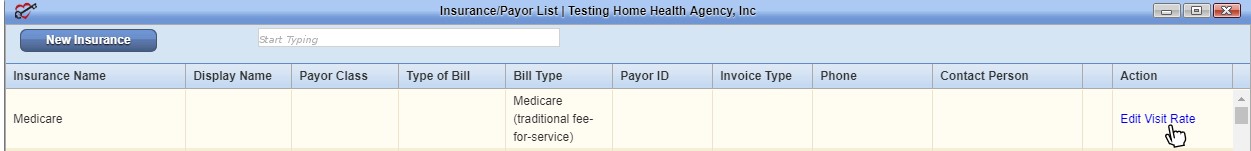
Selecting No next to Enable Pre-Claim Review (PCR) will remove this permission from the Permissions tab when adding or editing a new user.
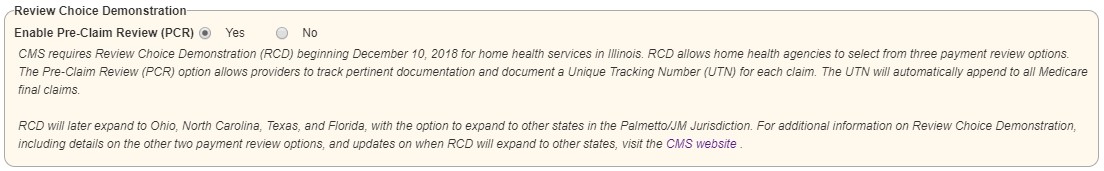
Leaving this option enabled will allow administrators to select users to manage PCR, in preparation for when RCD takes effect.
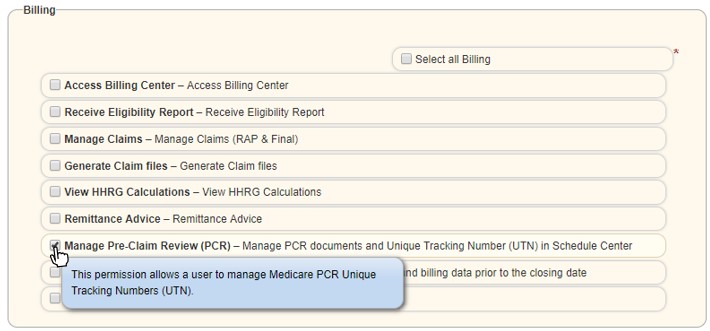
Enabling this permission will give the user access to a new Pre-Claim Review tab in the Schedule Center.
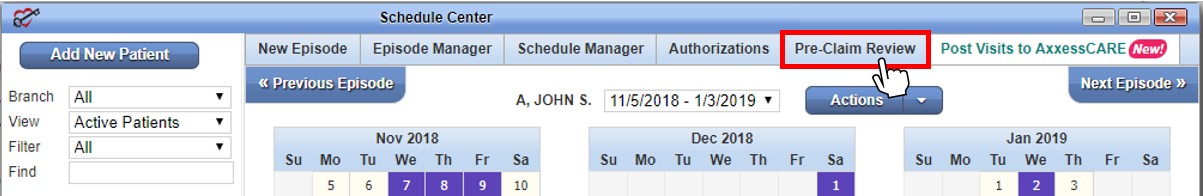
Selecting this tab opens the Manage Pre-Claim Review (PCR) Submissions page. Instructions for uploading and managing PCR documents are outlined on this page in three steps:
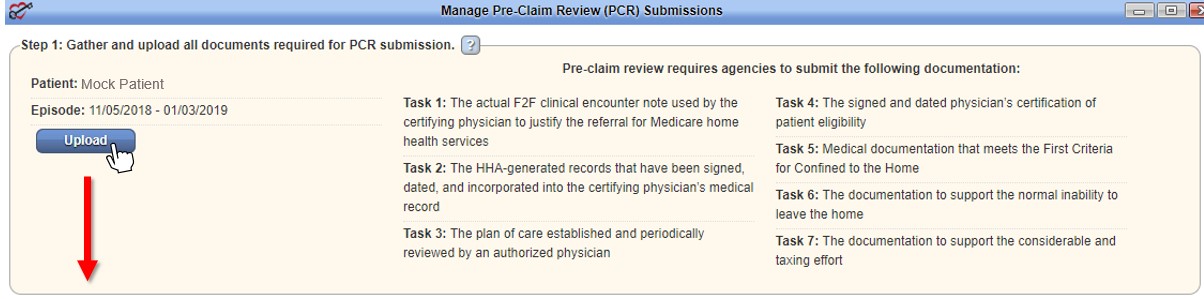
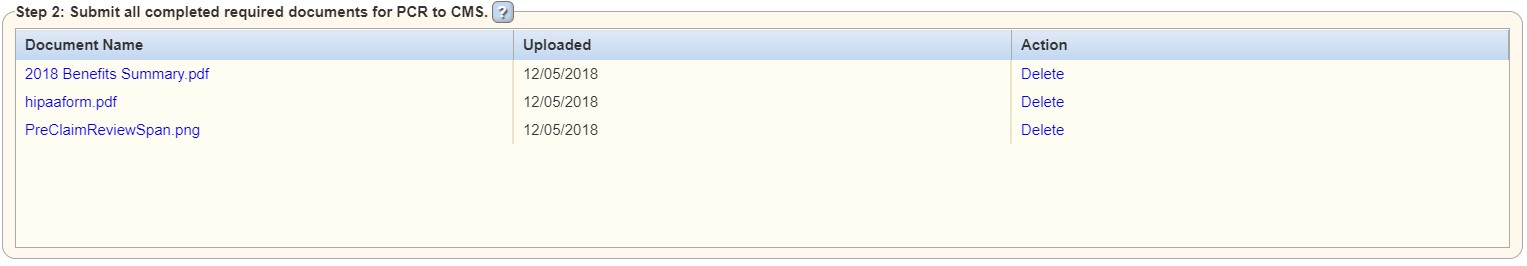
Uploaded documents will populate in the table on Step 2.
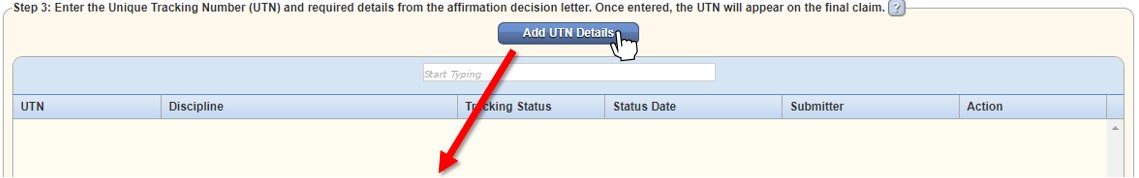
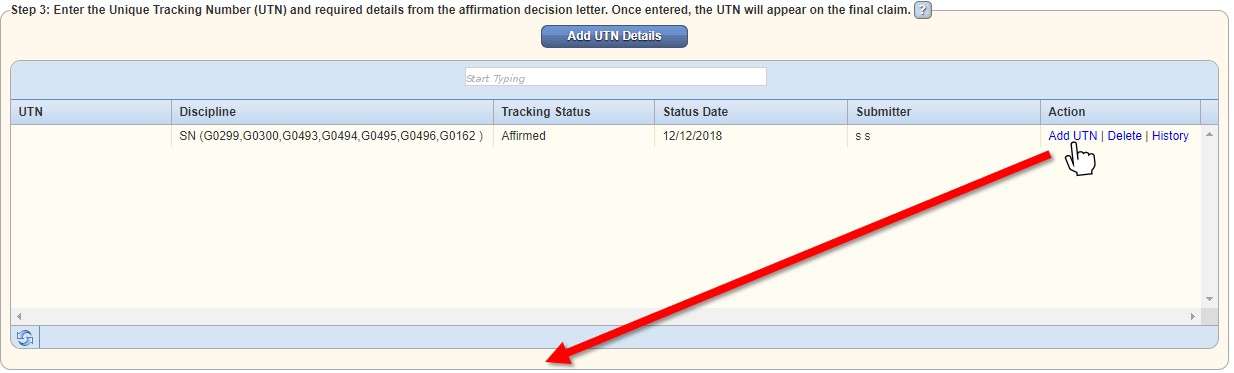
Once documentation has been submitted, the user can add tracking details by selecting Add UTN Details in Step 3.
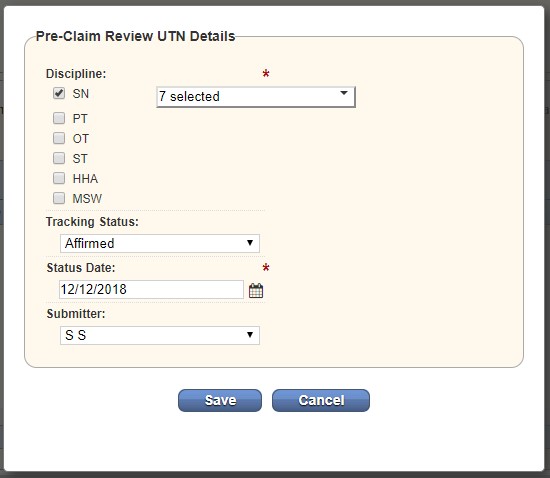
The method an agency uses to submit PCR documentation (fax, mail, or eServices) is the method that CMS will use to provide an affirmation decision letter. (If you choose to fax your PCR documentation, your affirmation decision letter will be faxed to you.)
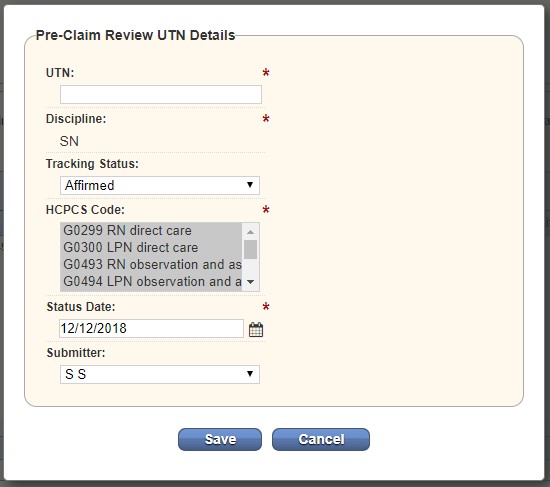
On receipt of your affirmation decision letter from CMS, add the UTN provided in the letter by selecting Add UTN under Action in Step 3.
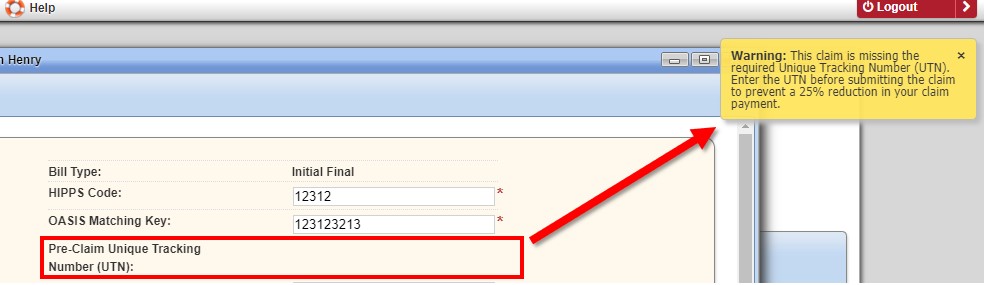
When a Medicare final claim is submitted without a UTN, a warning message will appear, reminding the user to enter the UTN before submitting the claim, to prevent a 25% reduction in claim payment. While waiting for RCD to take effect, users will continue to get this alert when a final claim is submitted without a UTN. The alert will not prevent users from submitting claims. Agencies can stop the warning (while waiting for RCD to take effect) by selecting Edit Visit Rate next to Medicare on the Insurances/Payors list and selecting No next to Enable Pre-Claim Review (PCR), as outlined above.
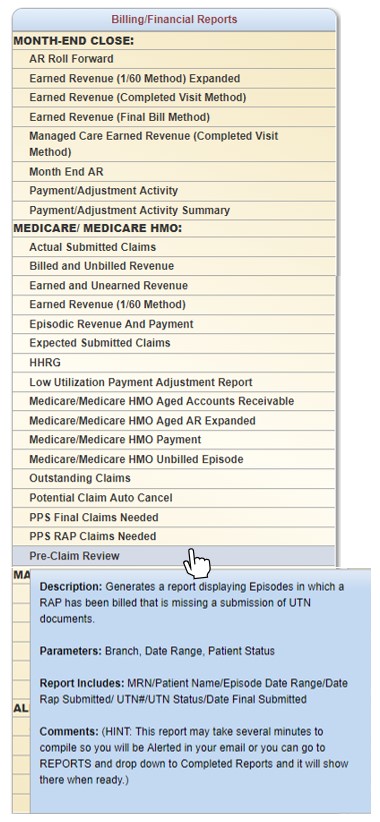
In the Report Center, a Pre-Claim Review Report is available under the Billing/Financial Reports section.
This report shows a summary of PCR information entered for each patient (in the parameters identified when requesting the report), including UTN, UTN Status, RAP Submission Date, and Final Submission Date. This report can be generated and used as a resource for agency leadership to review the PCR process and verify that claim submission information is being entered appropriately.
Important updates from CMS on Review Choice Demonstration are continuously posted to the CMS website.
Updated on 12/06/2018